A differentiated approach to cancer treatment designed to inhibit the activity of galectin-9
Our Approach
We are targeting novel, immunosuppressive and pro-tumor mechanisms to advance the treatment of a range of solid and hematologic malignancies
LYT-200: Clinical Stage Monoclonal Antibody Targeting Galectin-9
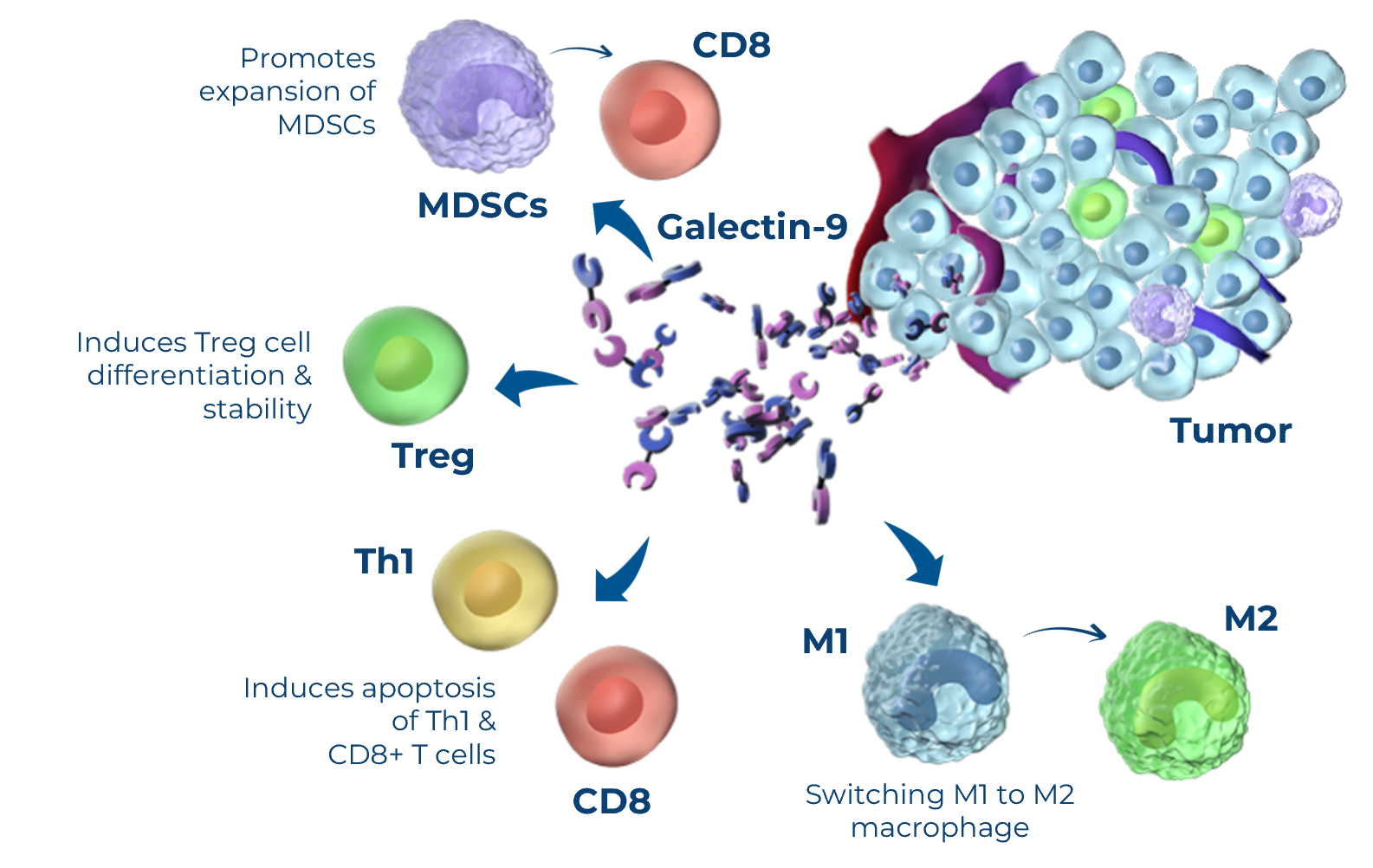
LYT-200 is an IgG4 monoclonal antibody targeting galectin-9, a protein secreted by tumor and immune cells that plays a key role in dampening the immune response against both solid and hematologic malignances. Galectin-9 expression is elevated in the tumor tissue and blood of patients with many types of malignancies, and increased levels correlate with more aggressive tumor characteristics and shorter survival. Galectin-9 has multiple immunosuppressive and pro tumor functions including down regulating multiple arms of the body’s immune response against cancer cells.
A large body of preclinical and human organoid data supports the potential clinical efficacy of LYT-200 and the importance of galectin-9 as a target. It is currently being developed for the treatment of metastatic/locally advanced solid tumors, including urothelial and head and neck cancers, as well as for the treatment of hematological malignancies, such as acute myeloid leukemia (AML). Phase 1b trials are currently ongoing in both solid tumors and AML/MDS.
See scientific publications at the bottom of this page
Galectin-9: Fundamental Immunosuppressor in Cancer
Galectin-9 modulates multiple pathways of cancer immunosuppression, including those involving by PD-1 and TIM-3
Gallop Pipeline
Metastatic Solid Tumors
Acute Myeloid Leukemia (AML)
Founded by PureTech Health (Nasdaq: PRTC, LSE: PRTC), Gallop Oncology was developed following the identification of galectin-9 as a promising oncology target with the potential to provide significant benefit to patients. Certain intellectual property was licensed from New York University prior to its publication in Nature Medicine..